An EHR/command and control system (including ordering, results reporting, etc.) for hospital Emergency Departments, Picis Pulsecheck, was recalled by FDA.
Reason? "Notes associated with prescriptions are not printed to the prescription or to the patient chart." The data apparently is not being sent to the printer or being stored for future visits. Instead, data input by clinical personnel, in one of the most risk-prone medical settings, the Emergency Department, is simply going away.
This is reminiscent of the truncation of prescription drug "long acting" suffixes, apparently by a Siemens system, that led to thousands of prescription errors (perhaps tens of thousands) over more than a year's time. I wrote about that matter, as reported by the news media, at "Lifespan (Rhode Island): Yet another health IT "glitch" affecting thousands - that, of course, caused no patient harm that they know of - yet" at http://hcrenewal.blogspot.com/2011/11/lifespan-rhode-island-yet-another.html.
Regarding the current Picis recall, notes connected with prescriptions can be crucial to the pharmacist or the patient. Loss of those notes - apparently due to a computer glitch and most likely in this case without the prescribing clinician knowing about it - likely have been going on for some time now, since two software versions (5.2 and 5.3) are affected.
The solution for now?
"Consignees were provided with recommended actions until they receive the necessary update."
In other words, a workaround adding more work to clinicians who now not only have to take care of patients, but in the unregulated health IT market need to (as if they don't already have enough work to do in the ED where chaos often occurs) babysit computer glitches as well - and pray they catch potential computer errors 100% of the time.
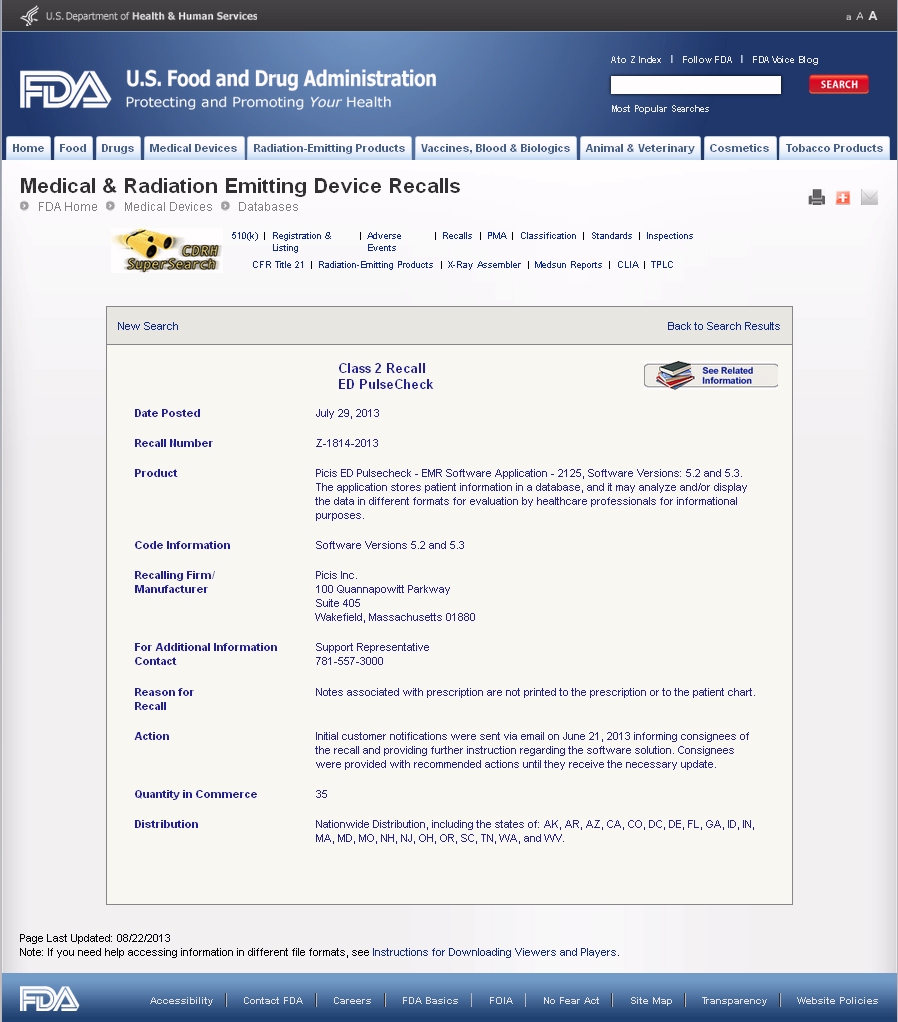
Below is the FDA MAUDE recall notice at "Medical & Radiation Emitting Device Recalls", from http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfRes/res.cfm?ID=119832.
At this additional link we find that this FDA recall was "Voluntary: Firm Initiated." They apparently informed the FDA of the "glitch."
My question is - how did the company become aware of this "glitch"? Also, were any patients put in harm's way, or injured, as a result of the prescription data loss?
 |
| FDA Device Recall Notice. Click to enlarge; text below. |
Finally, I ask - how did this "glitch" escape the notice of the company before the software was put into production not in just one, but through two sequential versions?
Class 2 Recall
ED PulseCheck
Date Posted July 29, 2013 Recall Number Z-1814-2013 Product Picis ED Pulsecheck - EMR Software Application - 2125, Software Versions: 5.2 and 5.3. The application stores patient information in a database, and it may analyze and/or display the data in different formats for evaluation by healthcare professionals for informational purposes. Code Information Software Versions 5.2 and 5.3 Recalling Firm/
ManufacturerPicis Inc.
100 Quannapowitt Parkway
Suite 405
Wakefield, Massachusetts 01880For Additional Information Contact Support Representative
781-557-3000Reason for
RecallNotes associated with prescription are not printed to the prescription or to the patient chart. Action Initial customer notifications were sent via email on June 21, 2013 informing consignees of the recall and providing further instruction regarding the software solution. Consignees were provided with recommended actions until they receive the necessary update. Quantity in Commerce 35 Distribution Nationwide Distribution, including the states of: AK, AR, AZ, CA, CO, DC, DE, FL, GA, ID, IN, MA, MD, MO, NH, NJ, OH, OR, SC, TN, WA, and WV.
I propose that the lack of health IT regulatory controls due to special accommodation makes thorough software testing less "desirable" by a company (largely due to costs).
Compare that to, say, software regulation in the Federal Aviation Administration:
 |
| FAA Aircraft Software Approval Guidelines - available at http://www.faa.gov/documentLibrary/media/Order/8110.49%20Chg%201.pdf. Click to access. |
The FAA document begins:
"This order establishes procedures for evaluating and approving aircraft software and changes to appropriate approved aircraft software procedures."
Software regulation in other mission critical industries like aviation and pharma make the health IT industry and its lack of regulation look pathetic.
-- SS


